While Ebola was primarily concentrated in Sierra Leone, Guinea, and Liberia, Zika has spread to over 62 countries and territories and WHO has declared it a global emergency1. Such declaration may push research to further understand the biology of the virus and governments to issue travel warnings, escalate pathogen surveillance, and increase demand for vaccines1. And although it may feel like Zika is a new anomaly within the infectious disease community, it’s history is long, and the warning signs may have been appearing long before the virus made headlines this year.
Going back to the roots: What is the history of the Zika virus?
 Zika isn’t new and has been around for decades, but is an emerging, neglected virus. Unfortunately, we know little about this virus. First isolated from a Rhesus macaque in the Zika forest in Uganda in 1947, Zika virus (ZIKV) is a single-stranded RNA arbovirus (of the Flaviviridae family, genus Flavivirus) transmitted by a variety of Aedes spp. mosquitos2,6. Since its identification, cases of Zika infections in humans have been reported sporadically with major epidemics occurring in 2013-2014 in French Polynesia, New Caledonia, the Cook Islands, and Easter Islands. Human travel to foreign lands may have aided the spread of the virus as unsuspecting ZIKV-infected humans travel to a location where the Aedes mosquitos thrive. The mosquito bites the infected human, in turn infecting the mosquito, which acts as a vector, unaffected by the virus. The infected mosquito bites another healthy human, transmitting the virus and the process continue
Zika isn’t new and has been around for decades, but is an emerging, neglected virus. Unfortunately, we know little about this virus. First isolated from a Rhesus macaque in the Zika forest in Uganda in 1947, Zika virus (ZIKV) is a single-stranded RNA arbovirus (of the Flaviviridae family, genus Flavivirus) transmitted by a variety of Aedes spp. mosquitos2,6. Since its identification, cases of Zika infections in humans have been reported sporadically with major epidemics occurring in 2013-2014 in French Polynesia, New Caledonia, the Cook Islands, and Easter Islands. Human travel to foreign lands may have aided the spread of the virus as unsuspecting ZIKV-infected humans travel to a location where the Aedes mosquitos thrive. The mosquito bites the infected human, in turn infecting the mosquito, which acts as a vector, unaffected by the virus. The infected mosquito bites another healthy human, transmitting the virus and the process continue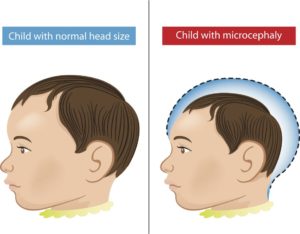 s as the vector continues to amplify and spread the virus.
s as the vector continues to amplify and spread the virus.
Global awareness primarily occurs if and when a virus turns into a global epidemic, which, in the case of Ebola and Zika, is exactly what happened. Particularly alarming with the recent Zika outbreak is the rate at which it has spread over time, and its link with Microcephaly and Guillain-Barré Syndrome in newborns2,3. Zika was linked to microcephaly when a sudden 20-fold increase in cases of microcephaly was observed among newborns in Brazil – suggesting a possible link2,3.
Continent Hopping: How did the Zika virus get to Brazil?
It is proposed that French Polynesians attending a cultural event introduced the Zika virus to Easter Islands, Chile, which then spread to Brazil and the Americas possibly through further human travel2. Baronti et al. (2014) report the complete coding sequencing of Zika virus recovered from a woman returning from the French Polynesia, who was hospitalized in France after experiencing fever, headache, and rash4. The team extracted viral RNA and used the Ion Torrent™ platform to analyze the genome of the virus4. A more recent case included that of a pregnant woman who returned from Brazil to Europe, showed signs of viral infection. During a routine ultrasound, it was observed that the unborn fetus had calcification in the brain and showed signs of microcephaly, she decided to terminate the pregnancy.
Mlakar et al. (2016) analyze the affected fetal tissues using Real-time PCR and Next-generation sequencing systems3. The Applied Biosystems™ 7500 Real-Time PCR was used to detect ZIKV transcripts in tested organs while the Ion PGM™ was used to recover the complete genome of this virus3. The real-time qPCR data showed ZIKV transcripts primarily concentrated in the brain, which was consistent with microscopy and immunofluorescence assays. Microscopy and immunofluorescence assays further indicated that the Zika virus replicated in the fetal brain. The recovered genome of this ZIKV had high identity with the strains isolated from French Polynesia (2013) and the strain from Sao Paulo, Brazil (2015)3. More so than the potential phylogeny of the ZIKV, what was interesting was the viral transcripts were primarily detected in brain tissue and were mostly absent from other organs – suggesting that the virus may be able to cross through the placenta into the fetus and also be able to cross the fetus’ blood-brain barrier. It is suspected that immune responses to the virus lead to calcification and neuronal damage in the studied fetus. Immune responses to neurotropic viruses (viruses that affect the central nervous system, such as Zika virus) may cause irreversible damage to the central nervous system5. We still need to know more about the pathology of the virus to understand the mechanism and association between Zika and Microcephaly or Guillain-Barré Syndrome.
Prevention is better than cure: Can we monitor the mosquitoes?
 When it comes to knowing if one is infected, some infected individuals may exhibit mild symptoms while other may be asymptomatic, making it even harder to detect visually or suspect an infection. How do officials detect virus spreading in a particular location when some people may not even show symptoms? The true extent of spread may remain a mystery until the mosquitos carrying the virus are examined. Traditional tests are serological and depend on the ability to detect specific antibodies or virus isolated from animals or mosquitos, which is a very time-consuming process6.
When it comes to knowing if one is infected, some infected individuals may exhibit mild symptoms while other may be asymptomatic, making it even harder to detect visually or suspect an infection. How do officials detect virus spreading in a particular location when some people may not even show symptoms? The true extent of spread may remain a mystery until the mosquitos carrying the virus are examined. Traditional tests are serological and depend on the ability to detect specific antibodies or virus isolated from animals or mosquitos, which is a very time-consuming process6.
Current sequencing methods or RT-qPCR techniques target one of the seven non-structural proteins, NS5, which is required for capping and synthesis of the viral RNA genome6. Faye et al. (2013) developed a real-time RT-PCR assay for detection of ZIKV (NS5 region) using an Applied Biosystems real-time PCR instrument , which can be performed in less than 3 hours, and the method was used to successfully detect ZIKV strains in field-caught mosquitos6. Fernandes et al. (2016) wanted to study the circulation of the virus in the Aedes mosquito itself, which is a vector for ZIKV7. They collected mosquitoes from several recreational parks from Sao Paulo, Brazil and using the Applied Biosystems™ 7500 Real-Time PCR studied the NS5 gene of the flavivirus. Their studies show a proof-of-concept method for surveillance of mosquitos carrying ZIKV in public places that may provide the perfect habitat for the mosquitos to grow7. Such rapid surveillance may help provide insights into the areas where infected mosquitos are present and how the virus circulates between the Aedes species, which in turn will help in generating awareness amongst local communities.
Breaking skin: How does the virus enter?
 Although studying the virus and the vector are important, understanding how the virus enters the host (humans/mammals) is equally important. Hamel et al. (2015) question ZIKVs infection and the signaling pathway involved as well as the host’s antiviral immune response8. These were some of the first studies that have explored the host-pathogen interaction and the biology of viral entry, replication, and the host’s immune response. The team studies ZIKV’s interaction with human skin cells, which is the first line of defense against the virus, using the Applied Biosystems™ 7300 Real-Time PCR instrument. They observe that some skin cell types like skin immune cells, including dermal fibroblasts, epidermal keratinocytes, and immature dendritic cells, are more permissive ZIKV infection. They also identify phosphatidylserine receptor AXL playing a major role in ZIKV entry receptor and enhancing ZIKV replication in cells that are permissive to infection. Viral replication activates an immune response and production of type I interferon in infected cells.
Although studying the virus and the vector are important, understanding how the virus enters the host (humans/mammals) is equally important. Hamel et al. (2015) question ZIKVs infection and the signaling pathway involved as well as the host’s antiviral immune response8. These were some of the first studies that have explored the host-pathogen interaction and the biology of viral entry, replication, and the host’s immune response. The team studies ZIKV’s interaction with human skin cells, which is the first line of defense against the virus, using the Applied Biosystems™ 7300 Real-Time PCR instrument. They observe that some skin cell types like skin immune cells, including dermal fibroblasts, epidermal keratinocytes, and immature dendritic cells, are more permissive ZIKV infection. They also identify phosphatidylserine receptor AXL playing a major role in ZIKV entry receptor and enhancing ZIKV replication in cells that are permissive to infection. Viral replication activates an immune response and production of type I interferon in infected cells.
We’ve only scratched the surface so far to understand the biology of the virus, the pathogenesis of Zika infection and its link to microcephaly or Guillain-Barré syndrome. But without current technology such as PCR, qPCR, or Next-generation-sequencing, it would be impossible for us to study rapidly emerging viruses that pose a threat to our society and existence. It is with this technology that we can dissect the biology of the virus, to understand how the virus enters the host, replicates, survives, targets organs, and if it causes microcephaly or other diseases. Future research questions will need to focus on how Zika enters into the fetal brain and affects the unborn, how the human immune system reacts to the virus, and a question some may have on their mind: If someone gets bitten by Zika, what is the possibility that the virus, which may lay dormant, can potentially affect future pregnancies.
Even with immense technological advances helping us today, I cannot stop but think about neglected diseases in general and why research in these areas is so un-supported, until it turns into a global crisis and then everyone scrambles for answers. Science and experimentation take time, especially when long term studies exploring the effects of viruses are required, which is a luxury we cannot afford during a global epidemic, where every moment counts. What is mind-boggling about Zika is that the 2013-2014 French Polynesia epidemic almost went unnoticed until it spread to Brazil. During the epidemic in French Polynesia, there were observations and questionable links of Zika to microcephaly or Guillain-barré. So, why did these observations get ignored, which could have possibly served as a warning sign? True, it’s always easier to look back and say – I wish, but I guess my question is – Must we always wait until an epidemic to understand infectious diseases?
Tell me your thoughts, What questions do you have? What are your concerns? Where do you think research should focus? Do you think we should focus more or less on neglected diseases?
Let me know in the comments section below!
All products are for research use only. Not for use in diagnostic purposes.
References:
1] Henderson, Barney, and Sarah Knapton. “Zika Outbreak Is Now a Global Emergency, Says World Health Organisation.” The Telegraph. Telegraph Media Group, 2 Feb. 2016. Web. 22 Feb. 2016.
2] Attar N: ZIKA virus circulates in new regions. Nat Rev Microbiol 2016, 14(2):62.
3] Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V et al: Zika Virus Associated with Microcephaly. N Engl J Med 2016.
4] Baronti C, Piorkowski G, Charrel RN, Boubis L, Leparc-Goffart I, de Lamballerie X: Complete coding sequence of zika virus from a French polynesia outbreak in 2013. Genome Announc 2014, 2(3).
5] Ludlow M, Kortekaas J, Herden C, Hoffmann B, Tappe D, Trebst C, Griffin DE, Brindle HE, Solomon T, Brown AS et al: Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol 2016, 131(2):159-184.
6] Faye O, Faye O, Diallo D, Diallo M, Weidmann M, Sall AA: Quantitative real-time PCR detection of Zika virus and evaluation with field-caught mosquitoes. Virol J 2013, 10:311.
7] Fernandes LN, Paula MB, Araujo AB, Goncalves EF, Romano CM, Natal D, Malafronte RD, Marrelli MT, Levi JE: Detection of Culex flavivirus and Aedes flavivirus nucleotide sequences in mosquitoes from parks in the city of Sao Paulo, Brazil. Acta Trop 2016, 157:73-83
8] Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F et al: Biology of Zika Virus Infection in Human Skin Cells. J Virol 2015, 89(17):8880-8896.

Leave a Reply