Lithium ion battery production
Over the past three decades, lithium-ion batteries have revolutionized the energy industry due to their lighter weight, longer charges and ability to perform better under extreme conditions compared to the nickel-cadmium batteries of the past. A key component of lithium-ion batteries is graphite, the primary material used for one of two electrodes known as the anode.
When a battery is charged, lithium ions flow from the cathode to the anode through an electrolyte buffer separating these two electrodes. This process is then reversed as the battery discharges energy. While various materials can be used for the cathode, graphite is the go-to material for most anodes, thanks to its abundance, low cost, and long cycle life. Cycle life refers to how long a battery can hold a charge and contributes to technology advancements.
Why is graphite used in batteries?
As industries around the globe work to create more powerful lithium-ion batteries to power everything from electric vehicles to grid-scale energy storage stations, graphite plays an increasingly important role. Natural graphite typically contains flakes which need to be converted to a spherical form before they can be used as an anode material. Alternatively, synthetic graphite can be produced in a controlled process to ensure consistent quality. The production of high-quality synthetic graphite requires temperatures as high as 3000°C.
Optimizing the morphology of the graphite allows researchers to create anodes with a higher rate capability and energy density, lower first cycle irreversible capacity loss, longer cycle life and better safety performance. Spherical graphite particles allow for more efficient packing of particles, thereby increasing the overall conductivity. Using a scanning electron microscope (SEM), researchers can visually study the morphology of the particles. Scanning electron microscopes have superior resolution compared to optical microscopes, which would prove difficult to use when looking at a black powder such as graphite.
SEM analysis of graphite for lithium ion batteries
Often, researchers can’t afford the in-house equipment or expertise and instead send their samples for analysis to testing labs with floor-model SEMs. Not only do these floor models require operation by a trained professional but outsourcing these analyses delays results.
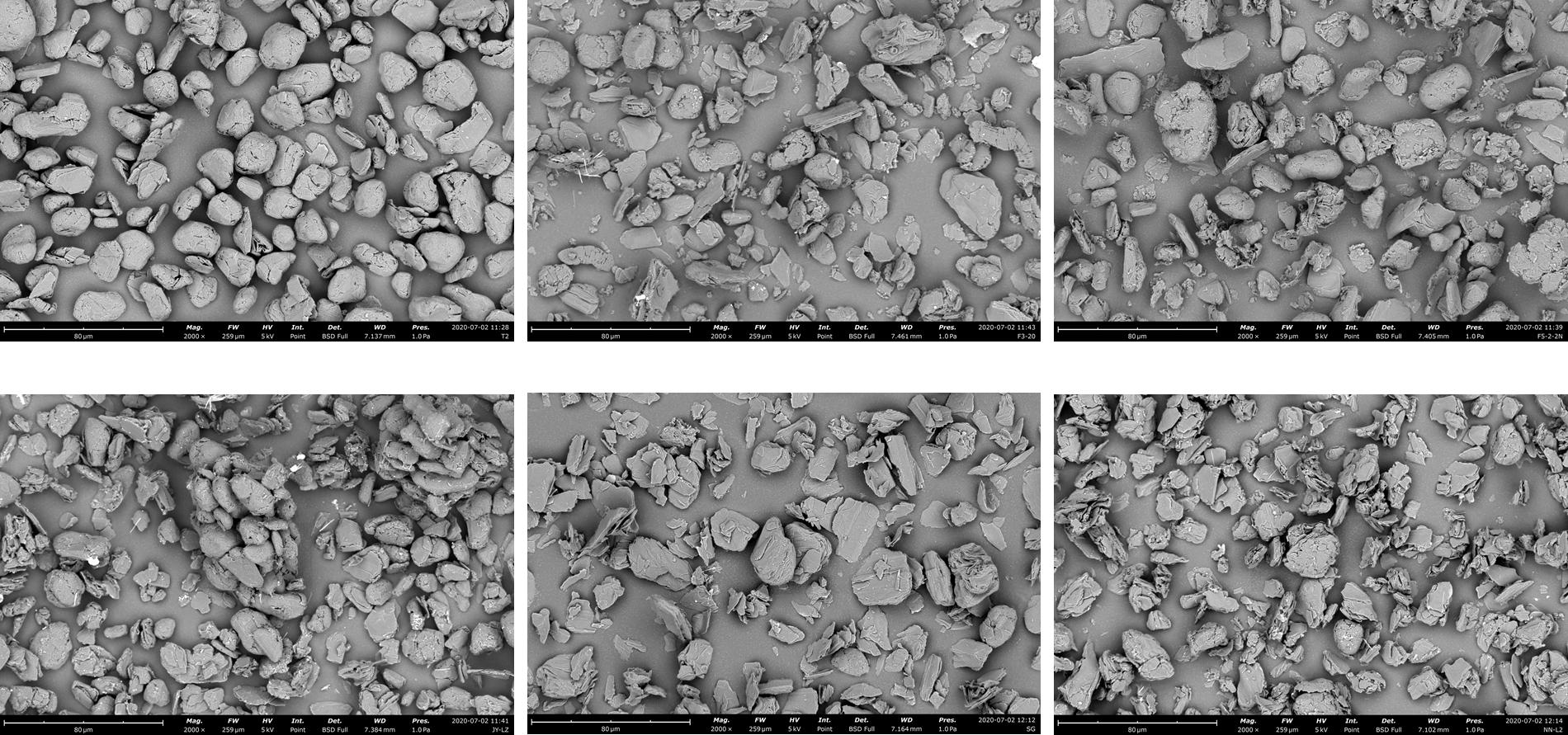
Graphite particle images captured using a Thermo Scientific Phenom XL Desktop SEM. The upper left-hand image is natural graphite. The other images were captured after applying five different treatments to synthetic graphite, with the goal of finding the closest match to the natural material.
By turning to the Thermo Scientific Phenom XL Desktop SEM with a dedicated Auto-Scan script, researchers can dramatically improve turnaround times for these types of battery experiments. The Phenom XL enables researchers to perform their own analyses in-house with very little training.
With the dedicated Auto-Scan script, the Phenom XL can automatically test up to 36 samples at the same time, generating more than 200 images in under 30 minutes. The script is easy to use: the user defines the number of positions that require analysis, and the desired magnification levels at all the positions. Once this is done, image acquisition can be done unattended overnight, dramatically reducing turnaround times while eliminating variations in image quality that are common with different operators.
The Phenom XL can generate images at 10 nanometer resolution, which makes it the ideal instrument to image 20-micron graphite particles. Moreover, the intuitive user interface reduces the need for training, extending these analyses to more users.
As scientists around the globe work to improve graphite for lithium-ion battery anodes, the Phenom XL Desktop SEM with dedicated Auto-Scan script can automate the repetitive testing work required. With the ability to quickly and accurately characterize these samples in-house, users can accelerate the R&D process as they work to design safer, more powerful, and longer lasting lithium-ion batteries.
Willem van Zyl is an application engineer at Thermo Fisher Scientific.
//
To learn more about how electron microscopy can enhance battery research, please see our Battery Research website.
Also, watch our on-demand webinar, Advanced Diagnostic Tools for Characterizing Lithium Metal and Solid-State Batteries.
Leave a Reply