Search Thermo Fisher Scientific
蛋白质谱分析综述
蛋白质质谱(MS)分析通过测量离子的质荷比,识别和定量分析简单和复杂混合物中的分子。MS 在很多领域和应用(包括蛋白组学)具有不可或缺的作用。最近二十年,高通量和定量 MS 蛋白组工作流程的发展扩展了我们在蛋白结构、功能、修饰和全基因组蛋白动力学领域的视野。本文概述了质谱在蛋白组学中的作用,回顾了质谱的方法及仪器,并涉及了一些在 MS 分析之前进行样本准备及利用液相色谱进行分离方面的知识。
蛋白质质谱简介
蛋白质组学 是在特定生物事件过程中针对一个生物系统(例如,细胞,组织)内所有蛋白进行的研究。由于蛋白表达的动态性,基因组学和蛋白组学的互补研究比基因组学研究更困难,甚至也比单独的转录组学研究更困难。此外大部分蛋白具有某种形式的转录后修饰(PTM),进一步增加了蛋白质组学的复杂性。在最近15年人们才开始认识到蛋白组学的广博,这很大部分得益于质谱技术的发展。
质谱是基于分子质荷比(m/z)对分子进行灵敏的检测、识别和定量的技术。MS 大约发明于 100 年前,当时用来测量元素原子量和特定同位素的自然丰度,它最初用于生物科学领域是对生物系统中痕量重同位素进行追踪;后来,MS 被用于对寡核苷酸和多肽进行测序,以及对核苷酸结构进行分析.
高分子电离方法发展,包括电喷雾电离(ESI)和大气压化学电离(APCI),促成了 MS 法蛋白质结构研究。 离子化还允许科学家们取得蛋白质质量 "指纹" ,可以将它们与数据库中的蛋白质和肽类进行匹配,从而帮助识别未知的靶标。最新的同位素标记方法更可支持对靶蛋白进行相对和绝对定量。上述技术进步共同催生出了一系列可成功分析固态、液态或气态样品的方法。质谱技术目前的灵敏度让人们可以在 attomolar 范围(10-18)内对分析物进行检测。
Mass Spectrometry Digital Resource Center
Improve your mass spectrometry results
Explore the new mass spec digital resource center to get practical information and tips to help you achieve your goals. Access the site to gain access to these free resources:
- Downloadable Thermo Scientific Protein Sample Preparation and Quantitation for Mass Spectrometry Handbook about tools and techniques for more robust and reproducible sample processing, protein quantitation and instrument calibration
- Helpful white papers and late breaking posters on specific applications such as subcellular fractionation, peptide fractionation, isobaric labeling and more
- On-demand webinars covering protein quantitation methods
蛋白质质谱应用
质谱分析测量离子的 m/z 比,识别和定量分析简单和复杂混合物中的分子。MS 在很多领域和应用(包括蛋白组学)具有不可或缺的作用。最近二十年,高通量和定量 MS 蛋白组工作流程的发展扩展了我们在蛋白结构、功能、修饰和全基因组蛋白动力学领域的视野。
本文概述了质谱在蛋白组学中的作用,回顾了质谱的方法及仪器,并涉及了一些在 MS 分析之前进行样本准备及利用液相色谱进行分离方面的知识。
所有质谱都有一个离子源、一个质量分析仪和一个例子检测器。这些组分的性质取决于质谱仪的类型、所需的数据类型以及样本的物理性质。样本可以以液态、气态或干粉形式加入到质谱仪中,然后气化并由离子源(如APCI, DART, EI)进行电离。

质谱仪的基本组分示意图。
这些分子所接受的电荷使得质谱仪在系统内对这些离子进行加速。这些离子受到来自质量分析仪的电场和/或磁场的影响,从而基于质荷比(m/z)改变其路径。常用的质谱仪包括飞行时间(TOF)质谱仪、Orbitraps、Quadrupoles和Ion traps质谱仪。质谱仪可以用来将全基因组分析的样本中的所有分析物分离出来,或者也可以用作一个过滤器,使得只有特定离子可以移动到检测器。
被质量分析仪改变路径的离子将撞击检测器。通常,这些检测器是电子扩增器或微通道板,当每个离子撞击检测板时将发射出级联的电子。这些级联电子对每个离子的撞击进行放大,提高了检测的灵敏度。上述整个过程都在极端真空(10-6 至 10-8 托尔)条件下进行(8)以避免气体分子、中子和非样本来源的污染离子和样本离子碰撞并改变它们的路径或产生非特异性反应产物。
最新的Orbitrap技术在一个中央纺锤形电极周围捕捉离子,然后在它们以不同的谐振频率通过纺锤形电极时分子其质荷比(m/z)。
质谱仪连接到基于计算机的软件平台,并在此测定离子振荡频率并通过图像电流检测获取质谱。数据分析程序检定离子并按照它们各自的 m/z 值 和相对丰度将它们进行编组。通过公认数据库确定这些离子,从而根据离子的 m/z 值预测该分子的同一性。
观看此视频,了解更多有关 Orbitrap Fusion 质谱仪的信息

一台扇形†质谱仪示意图。样品被注入到质谱仪中,分子被电离并加速。然后不同质量和电荷的离子被质量分析仪通过电磁场分离,轨迹正确的离子将被检测并且信号被放大。整个过程中系统处于高度真空状态。在信号放大之后,获得的数据将更具质荷比给出每个离子的相对丰度。†尽管扇形仪器由于质量分析仪的改进(如Quadrupole、Orbitrap技术)而使用减少了,但是这一简化的示意图给出了质谱的关键原理,即在复杂样品内选择和分析特定离子的能力。
串联质谱 (MS/MS)
可提供关于特定离子的额外信息。在这种方法中,受关注的不同离子在第一轮 MS 期间处于四极滤质器中(基于其 m/z ),并按照若干不同的解离方法进行裂解。其中一种方法是使用一束惰性气体对其进行碰撞,这被称为碰撞诱导的解离(CID)或更高能碰撞解离(HCD)。其它离子解离的方法包括电子转移解离(ETD)和电子捕获解离(ECD)。
这些解离后的片段在第二轮 m/z 分析中被根据其质荷比进一步分离。MS/MS 通常用来对蛋白和寡核苷酸进行测序,因为解离后的片段可以和 IPI、RefSeq 及UniProtKB/Swiss-Prot 等数据库的数据进行比对,预测多肽或核酸序列。这些序列片段通过 计算机模拟 编组,即可获得对于全长序列的预测。

串联质谱(MS/MS)示意图。样本被注入到质谱仪中,电离、加速并被质谱分析(MS1)。通过 MS1 的离子被选择性片段化然后在第二阶段的质谱仪分析(MS2)以获得离子片段的质谱数据。示意图显示的是分开的质量分析仪(MS1和MS2),但有些仪器使用同一质量分析仪进行两轮MS分析。
生物样本通常很复杂并包含可能掩盖目标分子的分子,例如当样本中的目标分析物和其它分子的浓度范围很广时。通常可以使用两种方法将目标分析物从样本内的其它分子中分离出来。通常可以使用两种方法将目标分析物从样本内的其它分子中分离出来。
气相层析(GC) 和 液相层析(LC)
气相色谱(GC) 和 液相色谱(LC)是使用 MS 分析复杂气体或液体样本时进行预分离的常用方法。LC-MS 通常用于分子热稳定性差和非易失性分子(如敏感的生物液体),而 GC-MS 被用于分析易失性化合物如石油化学制品。LC-MS 和 GC-MS 用来电离化合物的方法也不同。LC-MS 通常使用电喷雾电离(ESI),获得雾化的离子。GC-MS 可对样本进行直接电离或使用 ESI 进行间接电离。
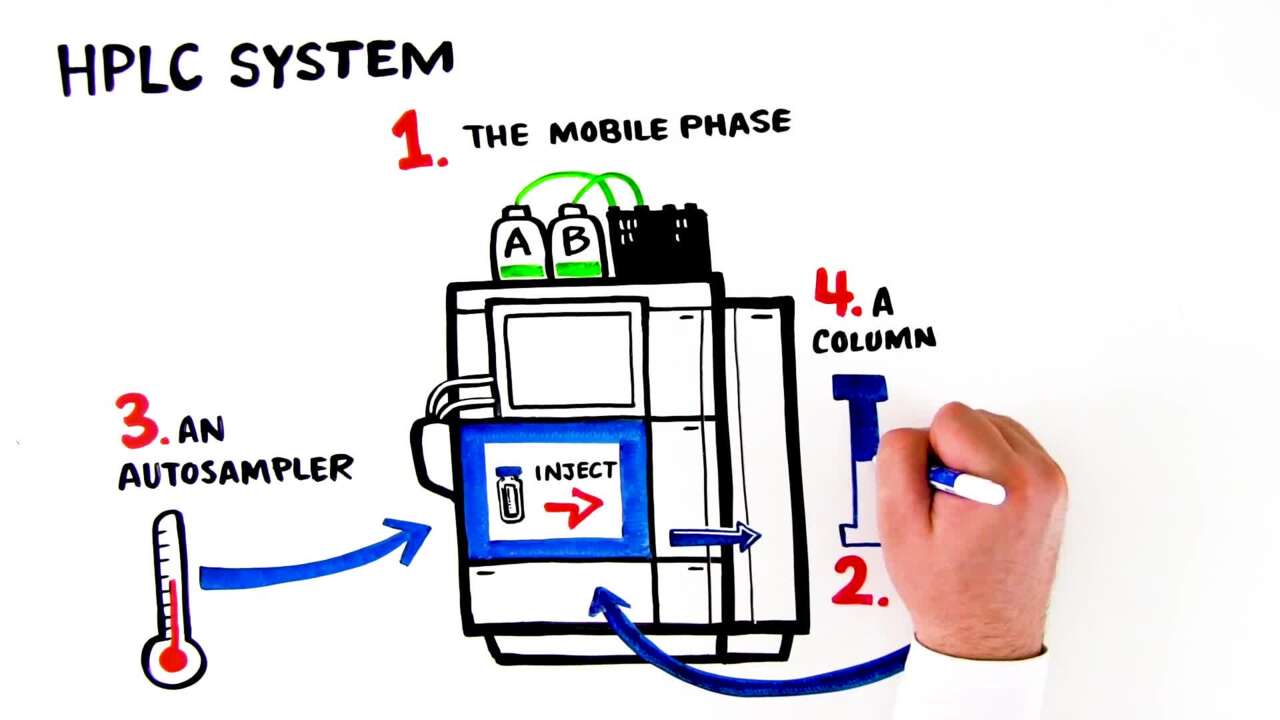
高效液相色谱(HPLC)
高效液相色谱(HPLC) 是使用 MS 或 MS/MS 分析生物样本时最常用的方法(分别被称为 LC-MS 或 LC-MS/MS),因为大部分生物样本是液态和非易失性的。LC 柱的直径很小(如 nanoHPLC 为 75 μm)并且流速很低(如 200 nL/min),非常适合分析微量样本。此外, "“串联”" 液相色谱(LC 直接连接到 Ms)提供了分析样本的高通量方法,使得从柱上以不同速率洗脱下来的多种分析物可以立刻被 MS 分析。例如,复杂混合物内的 1 到 5 个肽段可以使用串联 LC-MS/MS 进行测序。

LC-MS/MS 系统示例。Thermo Scientific Q Exactive Plus 和 Dionex UltiMate 3000 UHPLC系统。
UHPLC 简介
 How HPLC & UHPLC Instruments Work
How HPLC & UHPLC Instruments WorkThis video provides a brief history of chromatography and explains the basic principles behind how HPLC and UHPLC instruments work.
 Nano UHPLC Boosts Proteomic Research
Nano UHPLC Boosts Proteomic ResearchStephan Meding (Thermo Fisher Scientific, Application Scientist) discusses how new advancements made to the EASY-nLC 1200 system further optimize it for proteomics, producing better analytic depth, higher throughput and increased uptime.
 Viper Capillary Stainless Steel Fingertight Fittings
Viper Capillary Stainless Steel Fingertight FittingsThe Viper fingertight fitting system shows improved chromatographic results when compared with conventional capillary and fitting systems.
 Introducing the Thermo Scientific Vanquish UHPLC System
Introducing the Thermo Scientific Vanquish UHPLC SystemVideo on how the Thermo Scientific Vanquish UHPLC was developed and its features
 Wulff Nieder Introduces the UltiMate 3000 BioRS
Wulff Nieder Introduces the UltiMate 3000 BioRSFrom Pittcon 2013. Wulff Nieder Introduces the UltiMate 3000 BioRS.
了解更多信息
选择产品
定量蛋白质组学
虽然质谱可以在复杂混合物中检测到很低浓度的分析物,但它本身不能定量,因为在分析过程中相当多的肽段和离子损失。因此,需要同时对肽段标签或标准品进行分析,作为参考点进行相对或绝对定量。现在有商品化的产品能够实现在同一反应中对多个蛋白进行检测和定量,证明MS已经成为蛋白质组学领域内的高通量全基因组分析平台。
相对定量
相对定量 策略包括在细胞培养中使用氨基酸进行稳定同位素标记(SILAC)和串联质谱标记(TMT)。在这些方法中,蛋白和肽段被稳定同位素标记,从而获得不同于未标记分析物的独特质量变化。这一质量不同可以被 MS 检测并给出一个未标记分析物与标记分析物水平的比值。这些方法经常使用在以发现为目的的蛋白质组学中,使用不同大小的标记可以在很大的检测范围内找到很多蛋白。
绝对定量
绝对定量 用于在靶向蛋白质组实验中提高对有限数量的目标分析物进行检测的灵敏度。这些方法需要在样本中掺入已知量的含有重稳定同位素的合成肽段作为内源定量标准品,从而对样本中相应的天然肽段进行绝对定量。

SILAC 工作流程。对细胞培养物中的氨基酸进行稳定同位素标记(SILAC)须在缺乏赖氨酸和精氨酸的特制培养基中培养哺乳动物细胞。通过添加轻重形式的缺失氨基酸(例如,12C6 和 13C6 L-赖氨酸)可补偿这种缺陷。这方面的常规实验包括,在含有轻氨基酸培养基(对照)中培养一个细胞群,同时在含重氨基酸的培养基中(实验)培养另一细胞群。轻重氨基酸通过天然细胞蛋白质合成,进入蛋白质。在通过化学处理或遗传操纵改变一个样品中的蛋白质组后,将来自两个细胞群的等量蛋白质组合,通过 SDS 聚丙烯酰胺凝胶电泳(SDS-PAGE)分离并用胰蛋白酶消化,然后进行 MS 分析。
了解更多信息
选择产品
蛋白样品制备
所有样本在进行 MS 研究之前都需要进行一定形式的准备以便去除去垢剂以及降低样本的复杂性,从而专注于特定蛋白和/或标签蛋白以进行鉴定或定量。正确的样本制备对于 MS 分析十分关键,因为样本提取和制备的质量和可重复性显著影响从 MS 设备获得的结果。样本制备需要用到很多技术,包括裂解物制备、蛋白或肽段富集,以及样本纯化和蛋白消化。
观看此视频,了解更多有关质谱蛋白样品制备的信息
了解更多信息
选择产品
推荐阅读
- Kuster B 等著。(2005) 用蛋白质型肽探针对蛋白质组进行评分Nat Rev Mol Cell Biol 6:577–583.
- Mallick P, Kuster B (2010) Proteomics: A pragmatic perspective.Nat Biotechnol 28:695–709.
- Willard HH (1988) Instrumental methods of analysis.Belmont (CA): Wadsworth Pub.Co. xxi, p 895.
- Finehout EJ, Lee KH (2004) An introduction to mass spectrometry applications in biological research.Biochem Mol Biol Educ 32:93–100.
- Chowdhury SK 等著。(1990) Electrospray ionization mass spectrometric peptide mapping: A rapid, sensitive technique for protein structure analysis.Biochem Biophys Res Commun 167:686–92.
- Fenn JB et al.(1989) Electrospray ionization for mass spectrometry of large biomolecules.Science 246:64-71.
- Barber M et al.(1981) Fast atom bombardment of solids as an ion source in mass spectroscopy.Nature 293:270–5.
- Bakhtiar R, Tse FL (2000) Biological mass spectrometry: A primer.Mutagenesis 15:415–30.
- Hoffmann ED, Stroobant V (2001) Mass spectrometry: Principles and applications.Chichester (NY): Wiley. xii, p 407.
- Forsgard N 等著。(2010) 复杂基质中 14c 标记的生物活性化合物在阿摩尔浓度范围内的加速器质谱。Journal of Analytical Atomic Spectrometer 25:74–8.
仅供科研使用,不可用于诊断目的。