蛋白质通过静电作用(盐桥)、偶极作用(氢键、H 键)、熵效应(疏水作用)和分散力(碱基堆积)与 DNA 相互作用。这些力在不同程度上促进了序列特异性或非序列特异性蛋白结合。了解蛋白质如何与 DNA 相互作用,确定这些蛋白质– DNA 复合物中存在哪些蛋白质,以及确定组装这些复合物所需的核酸序列(和可能的结构),对于了解这些复合物在调节细胞过程中发挥的作用至关重要。为研究蛋白质与 DNA 的复杂相互作用,开发了许多实验室技术,每种技术都有其独特的历史、不同的用途和明显的优缺点。。
染色质免疫沉淀 (ChIP) 检测
染 色质免疫沉淀 (ChIP) 法可用于通过组蛋白修饰(表观遗传学)或转录因子– DN 结合相互作用监测转录调控。 ChIP 方法可通过甲醛或其他交联试剂处理细胞,分析活细胞中的 DNA 蛋白相互作用,以稳定用于下游纯化和检测的相互作用。进行 ChIP 检测需要了解 待 分析的靶蛋白和 DNA 序列。因为研究人员必须提供针对目标蛋白 的抗体和 PCR 引物用于 DNA 的序列。该抗体用于选择性地从其他基因组 DNA 片段和蛋白质 -DNA 复合物中沉淀蛋白质– DNA 复合物。PCR 引物可对目标 DNA 序列进行特异性扩增和检测。定量 PCR (qPCR) 技术 可以定量分析目标 DNA 序列的量。 ChIP 检测适用于基于阵列的格式(芯片上 ChIP)或对 免疫沉淀 蛋白捕获的 DNA 直接测序(ChIP-seq)。
优势 限制 捕捉特定蛋白与 DNA 的快照 耦合时的定量 qPCR 检测 能够为不同蛋白质绘制启动子图谱 研究人员需要采购 ChIP 级抗体 需要设计特定引物 难以适应高通量筛选
成功的染色质免疫沉淀 (ChIP) 实验的分步指南
更新的 ChIP 程序概述包括有关主要抗体选择(即 ChIP 验证抗体)的附加细节。该技术文档还描述并提供染色质免疫沉淀 (ChIP) 作为研究表观遗传学的技术的例子,因为它允许研究人员获得特定蛋白质-DNA 相互作用的快照。
我们的 72 页《蛋白相互作用技术手册》提供了实验方案,技术和产品信息,有助于最大化蛋白相互作用研究的结果。该手册可为 免疫沉淀 分析、共免疫沉淀 分析、沉降分析, 远红外免疫 印迹和交联背景,提供有用提示和故障排除建议。该手册还提供了一个关于 研究蛋白-核酸相互作用方法 的扩展部分,包括 ChIP,EMSA 和 RNA EMSA。该手册是任何实验室研究蛋白相互作用的重要资源。
内容包括:蛋白质相互作用简介,免疫共沉淀 分析,沉降分析,远 红外免疫印迹,蛋白质相互作用映射,酵母两杂交报告基因检测,电泳迁移率变动实验 [EMSA],染色质 免疫沉淀 检测 (ChIP),蛋白-核酸偶联物等。
蛋白相互作用手册
DN 电泳迁移率变动实验 (EMSA) 用于研究与已知 DNA 寡核苷酸探针结合的蛋白,并可用于评估相互作用的亲和力或特异性程度。技术的原理是,在非变性聚丙烯酰胺或 琼脂糖 凝胶上电泳时,蛋白质-DNA 复合物的迁移速度比游离 DNA 分子更慢。因为 DNA 迁移率会根据蛋白结合率发生偏移或速度减慢,因此,该实验也被称为凝胶偏移或凝胶延迟实验。在结合组分中添加蛋白特异性抗体会产生一个更大的复合物(抗体-蛋白– DNA), 这在电泳过程中的迁移速度甚至更慢。这被称为 “超迁移”, 并 可用来确认蛋白质特征。在 EMSA 形成之前,主要通过使用放射活性 标记探针的硝酸纤维素过滤柱结合试验,研究蛋白质– DNA 相互作用。
优势 限制 检测裂解物中的低丰度 DNA 结合蛋白 使用相同裂解物的许多探针配置检测结合位点突变 通过 DNA 探针突变分析测试结合亲和力 可采用 生物素化 或荧光标记的 DNA 探针的进行非放射性 EMSA 在体外分析蛋白与 DNA 的相互作用 难以定量 需要采用抗体进行 超迁移 检测,以确定复合物中的蛋白质特性
传统的DNA探针是经过³²P放射性标记的,其标记方法包括在使用 Klenow 片段进行 3'补平反应中加如一个[γ-³²P]dNTP ,或使用[γ-³²P]ATP 和 T4 多核苷酸激酶进行 5'末端标记。电泳后,将凝胶暴露于 X 射线胶片上以记录结果。 Thermo Scientific LightShift 化学发光 EMSA 试剂盒是一种非放射性测定,具有稳健、灵敏的性能。该试剂盒包括用于 设置和定制 DNA 结合反应的试剂、用于检测试剂盒系统的一组 DNA 和蛋白提取物、用于生物素标记 DNA 靶标的稳定链霉素亲和素–HRP 偶联物以及用于检测的一种极其灵敏的 化学发光 底物模块。
四种不同 DNA –蛋白 n 复合物的化学发光 EMSA。 生物素标记的靶标双链的大小范围为 21–25 bp。 OC-1,AP1 和 NF--κB 转录因子来源于 HeLa 核提取物。EBNA-1 提取物作为 LightShift 化学发光 EMSA 试剂盒中的对照品提供。存在未标记的特异性竞争序列(如使用),比标记靶标摩尔量高出 200 倍。每个系统的 X 射线胶片曝光时间:EBNA,Oct -1 和 AP1 分别为 2 分钟,NF-κB 为 5 分钟。
沉降检测用于从样品中选择性地提取蛋白质– DNA 复合物。通常,沉降检测使用带有高亲和力标签标记的 DNA 探针,如生物素,可回收或固定探针。 生物素化 DNA 探针可以 在与 EMSA 相似的反应中与细胞裂解物中的蛋白一起使用,然后用于纯化 琼 脂糖 EOR 磁珠的复合物。然后从 DNA 中洗脱蛋白质,并 通过质谱法检测到。或者, 可以用亲和标签标记蛋白质,也可以使用靶蛋白的抗体分离 DNA - 蛋白质复合物(与 超迁移 检测相似)。在这种情况下,通过 Southern 印迹或 PCR 分析检测结合蛋白的未知 DNA 序列。
优势 限制 富集低丰度靶标 可通过多种方法生成末端标记的 DNA 分离完整的复合物 与 免疫印迹 和质谱分析兼容 较长的 DNA 探针会 产生显著的非特异性结合 需要非常特异性的天然蛋白抗体 需要无核酸酶条件 必须在体外进行试验
微孔板 捕获检测法是 DNA 沉降检测法和 酶联 免疫吸附 检测法(ELISA)的混合方法,它使用固定的 DNA 探针捕获特定的蛋白质-DNA 相互作用,并用目标特异性抗体确认蛋白质的身份和相对数量。通常,将 生物素化 DNA 探针固定在涂有链霉亲和素的 96 孔或 384 孔 微孔板 表面。在结合缓冲液中制备细胞提取物,并添加足够多的时间让推定的结合蛋白与寡核苷酸结合。然后去除提取物,并对每个孔进行多次洗涤,以去除非特异性结合的蛋白质。最后,使用标记用于检测的特异性抗体检测蛋白。当使用酶标记抗体和 化学发光 底物时,这种方法的灵敏度极高,每孔可检测到小于 0.2 pg 的目标蛋白质。这种 微孔板 格式效率高,与高通量 检测兼容 ,可进行统计突变和激活检测。该方法还可用于使用其他标签标记的寡核苷酸,例如伯胺,可固定在涂有胺反应表面化学试剂的 微孔板 上。
优势 限制 使用基于 ELISA 的技术提高了速度和通量 与药物筛查兼容 可以优化灵敏的非放射性测定 需要对 DNA 结合天然蛋白(即 supershif 抗体)具有亲和力的抗体 数据仅提供了转录因子– DNA 亲和力或丰度的相对变化 检测试剂盒仅适用于少数靶标
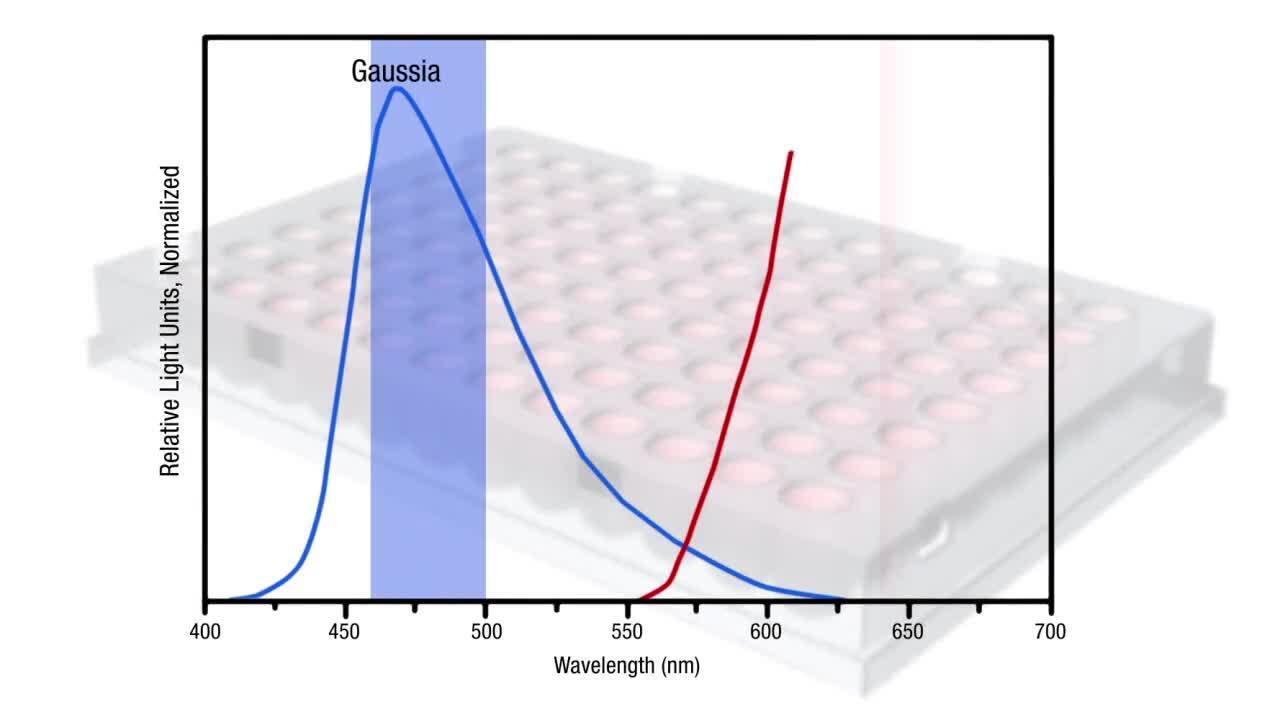
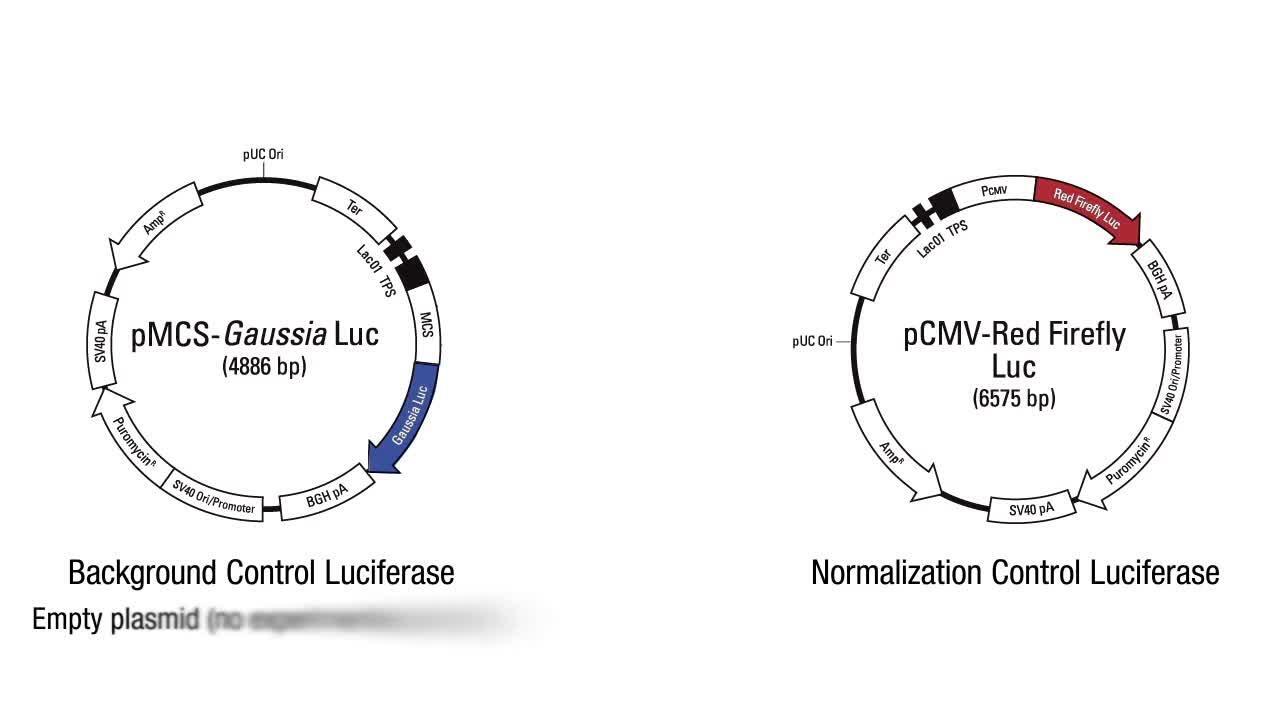
报告基因检测为目标启动子提供翻译活性的实时体内读数。报告基因是目标启动子 DNA 序列和报告基因 DNA 序列的融合。启动子 DNA 序列由研究人员定制,蛋白质的报告基因 DNA 序列编码具有可检测特性,如萤火虫荧光素酶、海肾荧光素酶或碱性磷酸酶。仅在目标启动子被激活时,这些基因才会产生酶。相反,该酶可催化底物,直至产生 光,颜色变化 或其他无法 通过光谱仪器检测的反应。报告基因的信号是翻译由同一启动子驱动的内源性蛋白的间接决定簇。
优势 限制 体内 监测捕获 实时数据 进行启动子突变分析的强大工具 适合高通量筛选 使用外源性 DNA 无法解决基因组序列引起的变化 可能出现基因剂量导致的伪影
观看视频,了解有关荧光素酶报告分析的更多信息
Video Player is loading.
Play Video Play Skip Backward Skip Forward Current Time 0:00
Duration 4:46
Seek to live, currently behind live LIVE Remaining Time - 4:46
Share Picture-in-Picture Fullscreen Beginning of dialog window. Escape will cancel and close the window.
Text Color White Black Red Green Blue Yellow Magenta Cyan Opacity Opaque Semi-Transparent Text Background Color Black White Red Green Blue Yellow Magenta Cyan Opacity Opaque Semi-Transparent Transparent Caption Area Background Color Black White Red Green Blue Yellow Magenta Cyan Opacity Transparent Semi-Transparent Opaque
Font Size 50% 75% 100% 125% 150% 175% 200% 300% 400% Text Edge Style None Raised Depressed Uniform Drop shadow Font Family Proportional Sans-Serif Monospace Sans-Serif Proportional Serif Monospace Serif Casual Script Small Caps Reset Done
Close Modal Dialog End of dialog window.
Close Modal Dialog This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Close Modal Dialog This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Video Player is loading.
Play Video Play Skip Backward Skip Forward Current Time 0:00
Duration 5:06
Seek to live, currently behind live LIVE Remaining Time - 5:06
Share Picture-in-Picture Fullscreen Beginning of dialog window. Escape will cancel and close the window.
Text Color White Black Red Green Blue Yellow Magenta Cyan Opacity Opaque Semi-Transparent Text Background Color Black White Red Green Blue Yellow Magenta Cyan Opacity Opaque Semi-Transparent Transparent Caption Area Background Color Black White Red Green Blue Yellow Magenta Cyan Opacity Transparent Semi-Transparent Opaque
Font Size 50% 75% 100% 125% 150% 175% 200% 300% 400% Text Edge Style None Raised Depressed Uniform Drop shadow Font Family Proportional Sans-Serif Monospace Sans-Serif Proportional Serif Monospace Serif Casual Script Small Caps Reset Done
Close Modal Dialog End of dialog window.
Close Modal Dialog This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Close Modal Dialog This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Video Player is loading.
Play Video Play Skip Backward Skip Forward Current Time 0:00
Duration 4:46
Seek to live, currently behind live LIVE Remaining Time - 4:46
Share Picture-in-Picture Fullscreen Beginning of dialog window. Escape will cancel and close the window.
Text Color White Black Red Green Blue Yellow Magenta Cyan Opacity Opaque Semi-Transparent Text Background Color Black White Red Green Blue Yellow Magenta Cyan Opacity Opaque Semi-Transparent Transparent Caption Area Background Color Black White Red Green Blue Yellow Magenta Cyan Opacity Transparent Semi-Transparent Opaque
Font Size 50% 75% 100% 125% 150% 175% 200% 300% 400% Text Edge Style None Raised Depressed Uniform Drop shadow Font Family Proportional Sans-Serif Monospace Sans-Serif Proportional Serif Monospace Serif Casual Script Small Caps Reset Done
Close Modal Dialog End of dialog window.
Close Modal Dialog This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Close Modal Dialog This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Video Player is loading.
Play Video Play Skip Backward Skip Forward Current Time 0:00
Duration 5:06
Seek to live, currently behind live LIVE Remaining Time - 5:06
Share Picture-in-Picture Fullscreen Beginning of dialog window. Escape will cancel and close the window.
Text Color White Black Red Green Blue Yellow Magenta Cyan Opacity Opaque Semi-Transparent Text Background Color Black White Red Green Blue Yellow Magenta Cyan Opacity Opaque Semi-Transparent Transparent Caption Area Background Color Black White Red Green Blue Yellow Magenta Cyan Opacity Transparent Semi-Transparent Opaque
Font Size 50% 75% 100% 125% 150% 175% 200% 300% 400% Text Edge Style None Raised Depressed Uniform Drop shadow Font Family Proportional Sans-Serif Monospace Sans-Serif Proportional Serif Monospace Serif Casual Script Small Caps Reset Done
Close Modal Dialog End of dialog window.
Close Modal Dialog This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Close Modal Dialog This is a modal window. This modal can be closed by pressing the Escape key or activating the close button.
Hendrickson W (1985) BioTechniques 3:346–354. Evertts AG et al.(2010) Modern approaches for investigating epigenetic signaling pathways.J Appl Physiol Jan 28 Epub ahead of print. Georges AB et al.(2010) Generic binding sites, deneric DNA-binding domains: Where does specific promoter recognition come from? FASEB Journal 24:346–356. Lunde BM et al.(2007) RNA-binding proteins: modular design for efficient function.Nat Rev Mol Cell Biol 8:479–490.