Search Thermo Fisher Scientific
生物标记和蛋白标记概述
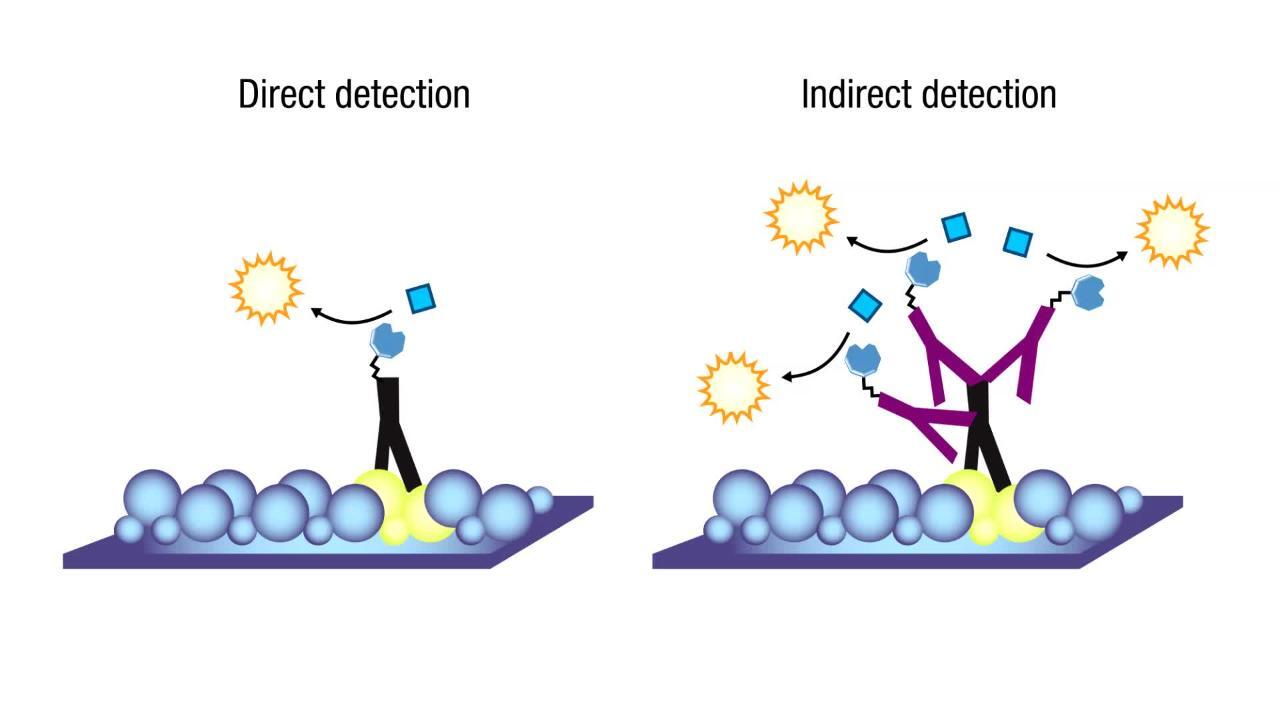
什么是蛋白标记?
蛋白标记物
生物素
生物素是一种用于蛋白质检测、纯化和固定的有用蛋白标记物,因为其与亲和素、链霉素亲和素或 Thermo Scientific NeutrAvidin 蛋白的结合能力非常强。事实上,这种相互作用是蛋白质和配体之间最强的非共价相互作用之一。此外,生物素(244.3 Da)比酶标记物小得多,因此不太可能干扰正常蛋白功能。总之,这些特性使亲和素-生物素策略成为许多检测和固定应用的理想选择。然而,根据应用性质,非常强的结合相互作用可能存在问题。在这些情况下,可获得亲和素或生物素衍生物的某些变体,可以软释放(洗脱)结合或可裂解(可逆)标记。
生物素化是用生物素分子标记蛋白或核苷酸的过程,可通过酶和化学方法进行。利用化学方法进行生物素化最为常用,用于这类标记的生物素化试剂具有几个共同的基本特征。它们由生物素基团、间隔臂以及负责与蛋白上靶官能团连接的反应基团组成。这三个特征的差异形成了多种可用试剂,并提供了特定应用所需的特殊性质。
间隔臂将生物素分子连接到与靶蛋白氨基酸上的某些官能团相互作用的反应基团上。除了将生物素连接到介导蛋白质连接的化学基团外,间隔臂还可通过三种方式影响生物素化和蛋白检测。首先,如下图所示,这些间隔臂长度不同,这会影响所连接的生物素与亲和素、链霉素亲和素或 NeutrAvidin 结合的可用性。

可变间隔臂长度的示例。化学基团(黑色)改变反应性部分(红色)和生物素(蓝色)之间的距离,以调节间隔臂的长度。所示的试剂为 (A) Thermo Scientific EZ-Link NHS-生物素,(B) EZ-Link NHS-LC-生物素以及 (C) EZ-Link Sulfo-NHS-LC-LC-生物素。
第二,生物素化试剂的溶解度是一个重要的因素,能够影响生物素化位于膜结合区室中蛋白的能力或改变标记靶蛋白的溶解度。例如,由聚乙二醇(PEG)重复组成的间隔臂将增加或保持标记蛋白的溶解性。

聚乙二醇可增加生物素化试剂的溶解度。四-乙二醇链(PEG4)偶联到反应性部分(红色)和生物素(蓝色)之间的间隔臂上。所示试剂为 Thermo Scientific EZ-Link NHS-PEG4-生物素。
相反,长的疏水性间隔臂可以使标记靶蛋白较不易溶解,但在疏水有机溶剂(如二甲基亚砜 [DMSO])中进行蛋白标记反应时较为理想,这在制备修饰疏水性肽时通常是必需的。第三,间隔臂可能含有可切割区域(例如,可还原的二硫键),其介导生物素标记与蛋白质的分离,以允许在没有苛刻变性剂的情况下进行纯化。
多种具有不同反应基团的生物素化试剂均有市售产品可供选择。常见的反应基团及其各自在蛋白质上的靶标包括:
- N-羟基琥珀酰亚胺(NHS)和磺基-NHS—伯胺
- 马来酰亚胺、碘乙酰基或吡啶基二硫化物—巯基
- 伯胺与 EDC 组合—羧基
- 肼和烷氧基胺—糖蛋白
此外,可光活化的芳基叠氮化物可用于在暴露于紫外光时介导非选择性生物素化。
生物偶联和交联技术手册
通过我们更新的生物偶联和交联技术手册,了解如何优化您的生物共轭策略。本指南简单易用,概述了我们用于蛋白质和肽的生物偶联、交联、生物素化和修饰的试剂组合。为您的典型应用实现最高效的修饰。
- 蛋白质和肽的生物素化
- 用荧光团和生物素标记抗体
- 将生物分子固定在表面上
- 捕捉蛋白质相互作用
继续阅读:生物素化
继续阅读:亲和素-生物素的相互作用
继续阅读:聚乙二醇(PEG)与蛋白质的聚乙二醇化
立即查看:生物素化
立即查看:生物素定量试剂盒
活性位点探针(酶作为标记靶标)
活性位点探针是一类化学标记试剂,其反应基团设计为特异性结合(标记)特定的酶活性位点。类似于传统的化学标记探针,活性位点探针包含可检测标签(生物素/染料)、间隔臂和负责连接到靶类酶的活性位点的反应基团。活性位点反应基团通常是与酶活性位点中的亲核残基共价连接的亲电化合物。在活性位点反应性基团不与靶酶共价结合的情况下,光反应性基团掺入连接子区域以促进特异性结合后的连接。这些探针可用于对样品中的靶标酶种类进行选择性富集、鉴定和谱分析,或评估酶抑制剂的特异性与亲和力。
已开发了活性位点探针来标记不同特定类别的酶,例如激酶、磷酸酶、GTP 酶、丝氨酸水解酶、半胱氨酸蛋白酶、金属蛋白酶和细胞色素 p450 酶。所有活性位点探针均可用于测定小分子对酶的抑制作用,部分探针还优先仅与活性酶反应,从而允许进行基于活性的蛋白质组学谱分析(ABPP)。与仅测量丰度的传统蛋白或 RNA 表达谱分析技术相比,ABPP 是一种监测蛋白活性的有效方法。下图和表说明了检测活性丝氨酸水解酶所涉及的过程,并分别提供了通过质谱法鉴定的丝氨酸水解酶列表。

丝氨酸水解酶活性位点探针的作用机制和化学结构。(A) 氟膦酸盐探针(FP)共价且特异性地结合至活性丝氨酸水解酶和蛋白酶的活性位点丝氨酸。(B) 用于活性丝氨酸水解酶的标记、亲和富集或荧光检测的叠氮基、脱硫生物素和荧光标记的氟膦酸盐探针的结构。
| 丝氨酸水解酶家族 | 鉴定的数量 |
|---|---|
| 水解酶 | 10 |
| 酯酶 | 6 |
| 脂肪酶 | 5 |
| 肽酶 | 4 |
| 其他 | 4 |
用 Activx 氟膦酸盐(FP)探针通过质谱法鉴定丝氨酸水解酶。使用脱硫生物素-FP 探针标记和富集后,通过质谱分析鉴定来自小鼠脑和肝组织提取物的丝氨酸水解酶家族成员的数量。
生物共轭技术,第 3 版
Greg T. Hermanson 所著的《生物共轭技术》 (第 3 版,2013 年)是对这本被公认为生物共轭领域权威参考指南的书籍的重大更新。
《生物共轭技术》是一本完整的教科书和操作手册,可供希望学习和掌握生物分子交联、标记和固定化技术的生命科学家使用,这些技术是许多实验室应用的基础。对于希望为全新应用开发新型共轭策略的研究人员来说,本书也是一本详尽而可靠的参考书。书中还广泛介绍了生物共轭领域,涵盖了该技术在不同科学学科中的所有主要应用,以及为任何目的设计最佳生物共轭物的技巧。
继续阅读:用于活性法酶分析的丝氨酸水解酶活性位点探针
立即查看:蛋白富集
立即查看:用于质谱分析的蛋白富集&纯化
酶偶联物(酶报告基因作为检测标签)
某些酶具有使其能够用作高灵敏度探针的特性,具有较长有效期,且在检测组织、全细胞或裂解物中的蛋白方面用途广泛。酶标记物比生物素大得多,且需要添加底物以产生可通过不同方法检测的显色、化学发光或荧光信号。酶标记物信号输出类型多、可实现信号放大且酶标记产物系列产品(特别是抗体)丰富,因此得到了广泛应用。
通常用作标记物的酶包括辣根过氧化物酶(HRP)、碱性磷酸酶(AP)、葡萄糖氧化酶和 β-半乳糖苷酶,每种酶都有特定的底物。事实上,HRP 和 AP 有多种市售底物,可产生比色、化学发光或荧光信号输出。为了生成以下数据,使用抗小鼠 IgG-HRP 偶联的二抗进行免疫组织化学(IHC)检测。

免疫组化法检测人肺结肠癌中 p21 的表达。使用单克隆抗体作为一抗以及抗小鼠 IgG-HRP 偶联物作为二抗,对人结肠癌的福尔马林固定石蜡包埋(FFPE)切片中的 p21 进行免疫组化染色。使用棕色沉淀的 HRP 底物 DAB。染色前,在10 mM 柠檬酸盐缓冲液中进行热诱导抗原决定簇修复(HIER)。
酶探针可以通过多种机制与抗体、链霉素亲和素或其他靶蛋白偶联,包括戊二醛、高碘酸盐氧化糖为活性醛后的还原胺化,或通过使用异双功能交联剂(如磺基-SMCC)。
观看此视频,了解更多有关将二抗用于免疫组化和其他应用的信息
继续阅读:酶探针
继续阅读:免疫组织化学(IHC)概述
立即查看:蛋白交联
立即查看:一抗
立即查看:IHC-免疫组织化学
荧光探针
不断开发出许多类型的用于染色或化学标记蛋白、核酸和其他生物分子的荧光试剂。当采用荧光染料对特异性抗体或其他纯化的生物分子进行化学生物标记时,它们成为适合检测靶抗原或相互作用伙伴的荧光探针,用于诸如细胞成像、高内涵分析、流式细胞分析、免疫印迹和 ELISA 等应用。
以下代表性实例包括使用多种荧光标记探针产生的免疫组织化学(IHC)和免疫细胞化学(ICC)数据,允许研究人员分别鉴定组织或细胞内的各种结构。

HeLa 细胞中脂联素的免疫细胞化学分析。使用 Invitrogen ABfinity 脂联素重组兔单克隆抗体进行该实验,然后使用 Invitrogen Alexa Fluor 488 偶联的山羊抗兔二抗(绿色)(A) 进行检测。细胞核用 DAPI 染色 (B),肌动蛋白用 Alexa Fluor 594 鬼笔环肽(红色)染色 (C)。图像 D 是显示核周区域中亚细胞定位的合成图像。

免疫荧光法检测人结肠癌组织中的细胞角蛋白 18。将切片与生物素化抗细胞角蛋白 18 抗体孵育,然后使用 Invitrogen 链霉素亲和素-DyLight 633 偶联物(红色荧光)进行检测。Invitrogen Hoechst 染料用于细胞核复染(蓝色荧光)。
荧光分子,也称为荧光基团或荧光染料,对光有直接和明显的响应,并产生可检测的信号。与酶或生物素不同,荧光标记物无需额外的检测试剂。这一特性使得荧光基团的用途极其广泛,并成为检测蛋白定位和活化、鉴定蛋白复合物形成和构象变化以及监测体内生物过程的新标准。
目前具有多种荧光基团可供选择,为研究应用提供了前所未有的灵活性、变化和性能。荧光基团可分为三大类,每一类探针都具有不同的特性。这些类别如下:
- 有机染料 — Alexa 染料、FITC、TRITC、Dylight 荧光染料
- 生物荧光基团 — 绿色荧光蛋白(GFP)、R-藻红蛋白
- 量子点
广泛的荧光试剂产品组合包括 Invitrogen Alexa Fluor 染料以及与 Invitrogen Molecular Probes 和 Thermo Scientific Pierce 产品系列相关的多种传统和专业荧光染料。
荧光探针的检测需要专门设备,包括激发光源、滤光片组和检测器,这些设备可在荧光显微镜、荧光孔板读数仪、流式细胞仪和细胞分选仪中找到。此类设备能够基于荧光对蛋白进行绝对定量分析,这是使用荧光探针优于其他类型探针的显著优点。

荧光的 Jablonski 能量图。当荧光基团被适当波长的光激发时,将吸收光,导致损失一些吸收的能量,同时发射更高波长的光,并最终返回分子的基态。
观看此视频,了解更多荧光基础知识
继续阅读:免疫组织化学(IHC)对比免疫细胞化学(ICC)
继续阅读:荧光探针
立即查看:荧光蛋白标记
立即查看:荧光基团选择
立即查看:SpectraViewer 荧光光谱查看器
立即查看:Molecular Probes 荧光大课堂
生物分子标记策略
已经开发了蛋白和核酸标记的 体外 和 体内 方法以适应所有类型的生物分子探针的需求。
体外标记
蛋白标记的化学方法包括使用能与特定氨基酸反应的化学基团偶联的生物标记物将标记物共价连接到氨基酸上。这些反应基团(在 Pierce 蛋白质研究方法库的交联剂章节中详细描述)与不同氨基酸上的特定部分反应,尽管也有少数可与 C-H 和 N-H 键上的任何氨基酸发生非特异性反应。这些反应基团也用于标记核酸。
酶法也用于标记蛋白和核酸。这些体外方法需要各自的聚合酶、ATP 和标记的氨基酸或核苷酸。虽然体外 DNA 转录相对直接,但通过体外翻译表达标记的蛋白可能较为困难,因为需要适当的蛋白长度、折叠和翻译后修饰,而一些市售试剂盒无法提供这些特性。

通过使用 T4 RNA 连接酶和 ATP,使用生物素化胞苷二磷酸(pCp-生物素)在3'羟基端对 RNA 进行酶标记。
体内标记
代谢标记是通过分别用标记的核苷酸或氨基酸培养细胞来标记细胞中的所有核酸或蛋白的方法。在含有标记核酸或氨基酸的培养基中延长细胞培养可使所有 DNA、RNA 或蛋白通过 DNA 复制、翻译和蛋白周转(protein turnover)而被标记。然后可以纯化关注的核酸或蛋白用于进一步实验。进行代谢标记的好处在于所有核酸或蛋白种类可以实现一致标记。相反,代谢标记可能有毒,取决于使用的生物标记物类型,并且代谢标记试剂的数量并不像 体外 方法那样广泛。

几种体内交联方法的比较。淬灭前,用1%甲醛(HCHO)或1 mM 同双功能 NHS-酯交联剂(Thermo Scientific Pierce DSG 和 DSS)的 PBS 溶液处理 HeLa 细胞10分钟。根据实验方法,用4 mM 光反应-亮氨酸、2mM 光反应-甲硫氨酸(photo-AA)对第四组 HeLa 细胞进行处理并交联10分钟。分别用100 mM 甘氨酸(pH 3)和500 mM Tris(pH 8.0)对经甲醛处理和经 NHS-酯处理的细胞进行额外淬灭15分钟。然后将每种实验条件下的1百万个细胞裂解,并将10 μg 每个样品于 65°C 下在含50 mM DTT 的还原缓冲液中加热10分钟,然后使用 SDS-PAGE 和 Stat3 特异性抗体进行免疫印迹分析(细胞信号转导)。使用 GAPDH(Santa Cruz)和 β-actin(US Biologicals)作为内参进行免疫印迹分析。
推荐阅读
仅供科研使用,不可用于诊断目的。